Al3+ Ion Electron Configuration
The electron configuration for Aluminum is 1s2 2p2 3s6 3p1. In this video we will write the electron configuration for Al 3 the Aluminum ion.

Al 3 Electron Configuration Aluminum Ion Youtube
The nex six electrons will go in the.

. Up to 256 cash back Get the detailed answer. Electron Configuration for Fe Fe2 and Fe3 Iron and Iron Ions In writing the electron configuration for Iron the first two electrons will go in the 1s orbital. Al 13 1s2 2s2 2p6 3s2 3p1 or Ne10 3s2 3p1.
The Al3 ion is produced by the decrease in electrons in aluminium metal so Al3 ion consists of only ten electrons in it and its outer shell being similar to neon. Examples of isoelectronic species are N 3 O 2 F Ne Na Mg 2 and Al 3 all have the electron configuration 1s 2 2s 2 2p 6. 4 pts Write the full electron configuration for the Al3ion.
So yes Al3 has the same. Since 1s can only hold two electrons the next 2 electrons for Iron go in the 2s orbital. The electronic configuration of Al 3 is 1 s 2 2 s 2 2 p 6.
In writing the electron configuration for Aluminium the first two electrons will go in the 1s orbital. That is aluminum is a cation element. This electron configuration shows that aluminum ion Al 3 has acquired the electron configuration of neon and it achieves an octave full stable electron configuration.
Welcome to our channel K2chemistryclass This is an educational Channel specially for chemistryElectronic configuration full video-https. The electronic configuration for Al metal is as follows. Since 1s can only hold two electrons the next 2 electrons for aluminium go in the 2s orbital.
In this video we will write the electron configuration for Al 3 the Aluminum ion. The p orbital can hold up to six electrons. The next six electrons will go in the 2p orbital.
When determining the electron configuration of an ion for main group elements the electrons that were added last are the ones that are removed first when forming a cation. Thus it has total 10 valence electrons. But for the Ion which is Al 3 it has a positive chargetherefore electrons need to be taken away not added also the octet rule must.
When the Aluminium Al atom loses three electrons it forms Al 3 ion. Al 3e Al 3. So in the 3 oxidation state aluminium attain the noble gas electronic configuration.
Lewis dot structure of Al. 3 pts Which has a larger radius Al. Well also look at why Aluminum forms a 3 ion and how the electron confi.
Well also look at why Aluminum forms a 3 ion and how the electron confi. The electron configuration of aluminum ion Al 3 is 1s 2 2s 2 2p 6. Electron configuration al3 LIMITED TIME OFFER.
GET 20 OFF GRADE YEARLY SUBSCRIPTION. View the full answer. When aluminum Al 13 reacts it loses its three valence electrons to achieve the same electron configuration as neon Ne 10 That configuration is 1s22s22p6.
Electronic configuration of Al 3. 3 pts Name a neutral atom which shares the same electron configuration as Al3 ion. It is formed by losing 3.
Its electronic configuration is has 10 electrons and 13 protons. When we write the configuration well put all 13 electrons in orbitals around the nucleus of the Aluminium atom.

Electronic Configuration Of Al Al3 Youtube

Al 3 Electron Configuration Aluminum Ion Youtube
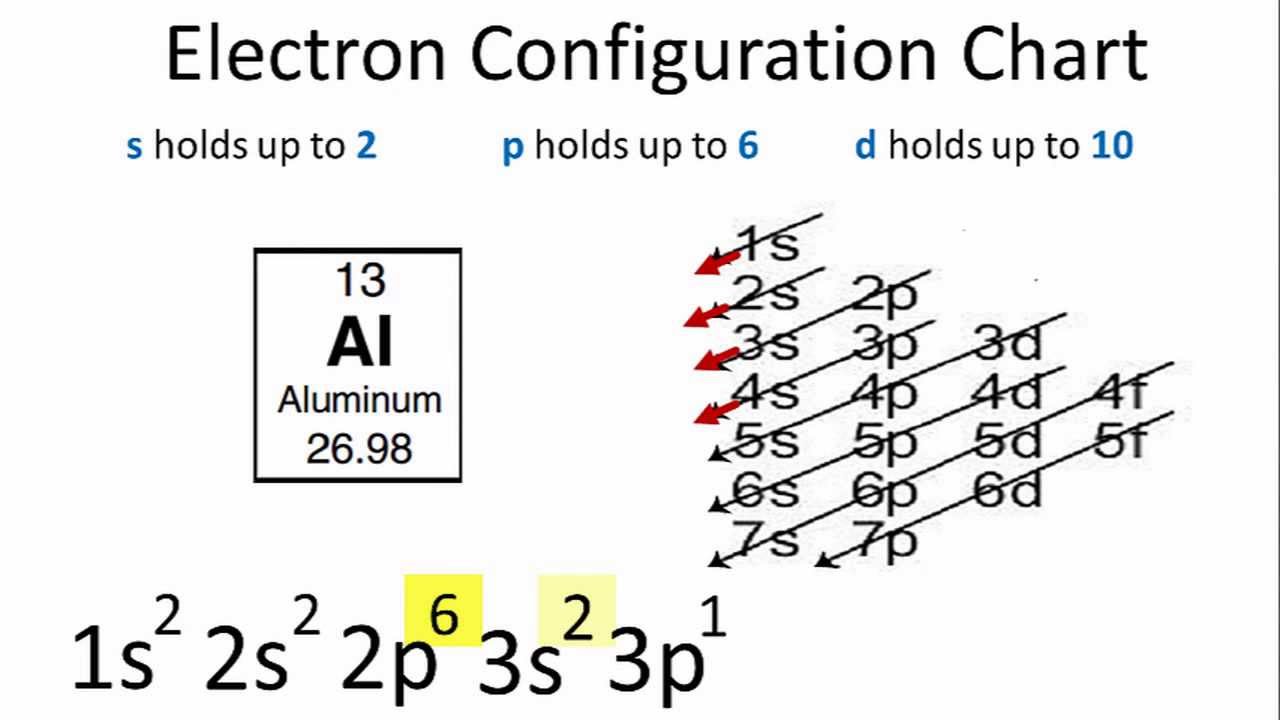
Electron Configuration For Aluminium Al

Electron Configuration For Aluminium Al
0 Response to "Al3+ Ion Electron Configuration"
Post a Comment